
您当前的位置:首页 » 产品展示 » 色谱柱 » Porapak Qs 无菌医疗器械中环氧乙烷的残留测定填充柱

| 产品名称: | Porapak Qs 无菌医疗器械中环氧乙烷的残留测定填充柱 |
| 产品型号: | Porapak Qs |
| 品牌: | 1356 |
| 产品数量: | |
| 产品单价: | 面议 |
| 日期: | 2022-05-03 |
Porapak Qs 无菌医疗器械中环氧乙烷的残留测定填充柱的详细资料
无菌医疗器械中环氧乙烷的残留测定填充柱无菌医疗器械中环氧乙烷的残留测定填充柱 详细信息:
名称:填充柱
固定相:高分子小球
粒度:60-80目
规格:1-2m*内径2-3mm
型号:Porapak Qs
应用:
GB 19335-2003 一次性使用血路产品 通用技术条件
GB14233.1-2008-T 医用输液、输血、注射器具检验方法 第1部分:化学分析方法
《GBT33418-2016-环氧乙烷灭菌化学指示物检验方法》
GB 15979-2002 一次性使用卫生用品卫生标准
GBT 14233.1-1998 医用输液、输血、注射器具检验方法 第1部分:化学分析方法
GB-T16886.7-2015医疗器械生物学评价第7部分环氧乙烷灭菌残留量
SNT 4391-2015 一次性卫生用品 卫生巾、卫生护垫、纸尿裤、消毒棉 环氧乙烷残留量的测定
随着对医疗器械的规范管理,对于用环氧乙烷灭菌的无菌医疗器械,其残留量应该被重点关注。
浩瀚色谱(山东)应用技术开发有限公司介绍了影响环氧乙烷残留量的因素、残留量的检测、日常抽样、放行等问题,并对目前企业存在的问题进行了分析。
无菌医疗器械中环氧乙烷的残留测定填充柱 测试谱图:
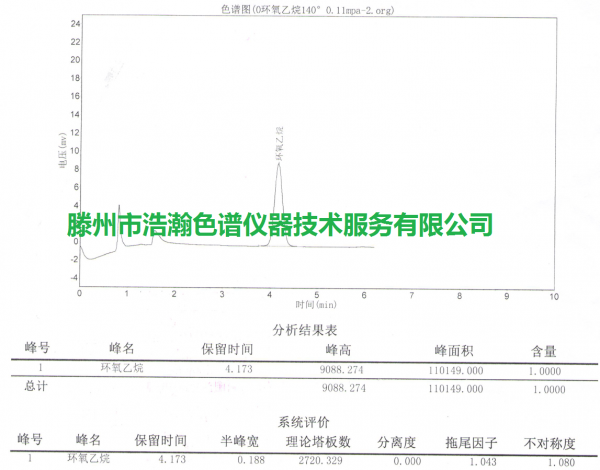




Packed column for the determination of ethylene oxide residues in sterile medical devices
Packed Columns for Residue Determination of Ethylene Oxide in Sterile Medical Devices Details:
Name: Packed Column
Statio
Granularity: 60-80 mesh
Specifications: 1-2m* inner diameter 2-3mm
Model: Porapak Qs
application:
GB 19335-2003 One-time use of blood circuit products General technical conditions
GB14233.1-2008-T Test method for medical infusion, blood transfusion and injection equipment-Part 1: Chemical analysis method
"GBT33418-2016-Test method for chemical indicators of ethylene oxide sterilization"
GB 15979-2002 Hygiene Standard for Disposable Hygiene Products
GBT 14233.1-1998 Test methods for medical infusion, blood transfusion and injection equipment - Part 1: Chemical analysis methods
GB-T16886.7-2015 Biological e
SNT 4391-2015 Disposable sanitary products - Sanitary napkins, sanitary pads, diapers, disinfection cotton - Determination of ethylene oxide residues
With the standardized management of medical devices, the residual amount of sterile medical devices sterilized with ethylene oxide should be the focus.
Haohan Chromatography (Shandong) Application Technology Development Co., Ltd. introduced the factors affecting the residual amount of ethylene oxide, the detection of residual amount, daily sampling, release and other issues, and analyzed the current problems of the enterprise.
Packed column for the determination of ethylene oxide residues in sterile medical devices Test chromatogram: