
产品展示
您当前的位置:首页 » 产品展示 » 色谱柱 » Chromosorb 101 HP 60~80填充柱 测定一次性使用无菌注射器中环氧乙烷残留量

| 产品名称: | Chromosorb 101 HP 60~80填充柱 测定一次性使用无菌注射器中环氧乙烷残留量 |
| 产品型号: | Chromosorb 101 HP 60~80填充柱 |
| 品牌: | 1356 |
| 产品数量: | |
| 产品单价: | 面议 |
| 日期: | 2022-05-01 |
Chromosorb 101 HP 60~80填充柱 测定一次性使用无菌注射器中环氧乙烷残留量的详细资料
测定一次性使用无菌注射器中环氧乙烷残留量测定一次性使用无菌注射器中环氧乙烷残留量 详细信息:
Chromosorb 101 HP 60~80目;玻璃柱长2m,f 3 mm
材质:不锈钢
应用:GB 15979-2002 一次性使用卫生用品卫生标准
本产品严格按照一次性使用无菌注射器国际标准(GB15810-1995)生产,采用医用高分子材料在十万级净化车间加工而成,经环氧乙烷灭菌,无毒,无菌,无热源.本产品已作过无菌毒性,热源测试,安全有效可直接使用.
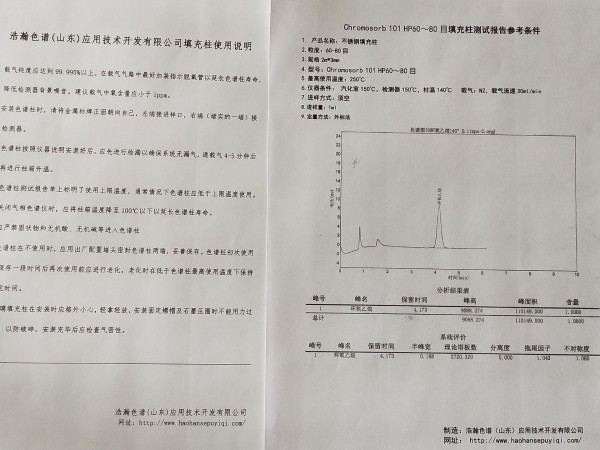
浩瀚色谱(山东)应用技术开发有限公司建立测定一次性使用无菌注射器中环氧乙烷残留量的顶空气相色谱方法。方法采用Chromosorb 101 HP 60~80目2m*3mmm色谱柱,FID检测器,进样口温度200℃,柱温45℃,检测器温度200℃,顶空平衡温度80℃,保温30 min。结果环氧乙烷在1.0~10.0μg/mL范围内呈良好的线性关系(r=0.999 1),检出限为0.04μg/mL,平均加样回收率为98.85%(n=9),重复性试验相对标准偏差(RSD)为2.84%(n=6)。结论该方法灵敏、准确、重复性好,可用于一次性使用无菌注射器的临床安全性评价。
测定一次性使用无菌注射器中环氧乙烷残留量 测试谱图:










Determination of Residues of Ethylene Oxide in Single-Use Sterile Syringes
Determination of Residues of Ethylene Oxide in Single-Use Sterile Syringes Details:
Chromosorb 101 HP 60~80 mesh; glass column length 2m, f 3 mm
Material: Stainless steel
Application: GB 15979-2002 Hygiene Standard for Disposable Hygiene Products
This product is produced in strict accordance with the internatio
Haohan Chromatography (Shandong) Application Technology Development Co., Ltd. has established a headspace gas chromatography method for the determination of ethylene oxide residues in disposable sterile syringes. Methods Chromosorb 101 HP 60~80 mesh 2m*3mmm chromatographic column, FID detector, inlet temperature 200℃, column temperature 45℃, detector temperature 200℃, headspace equilibrium temperature 80℃, and the temperature were kept for 30 min. Results Ethylene oxide showed a good linear relatio
Determination of ethylene oxide residues in disposable sterile syringes Test chromatogram: