
产品展示
您当前的位置:首页 » 产品展示 » 色谱柱 » Porapak QS填充柱 生药含水量的气相色谱测定法

| 产品名称: | Porapak QS填充柱 生药含水量的气相色谱测定法 |
| 产品型号: | Porapak QS填充柱 |
| 品牌: | 1356 |
| 产品数量: | |
| 产品单价: | 面议 |
| 日期: | 2025-02-12 |
Porapak QS填充柱 生药含水量的气相色谱测定法的详细资料
生药含水量的气相色谱测定法生药含水量的气相色谱测定法 详细信息:
名称:填充柱
规格:2m*3mm
型号:Porapak QS
应用:生药含水量的气相色谱测定法
药物中水分含量的多少直接影响药物的质量。为此药典对许多生药、合成药或制剂规定了含水量限度。生药含水量测定,我国药典采用重量法(或称干燥失重)和甲苯法(又称共沸法)。国外药典有关项下与我国药典方法基本一致,仅在某些操作细节上略有差异。实际工作中,这两种方法存在着取样量大、费时及操作繁杂等缺点。
滕州市浩瀚色谱仪器技术服务有限公司,,采用Porapak QS填充柱测定生药含水量的,柱效高,分离好,结果满意。
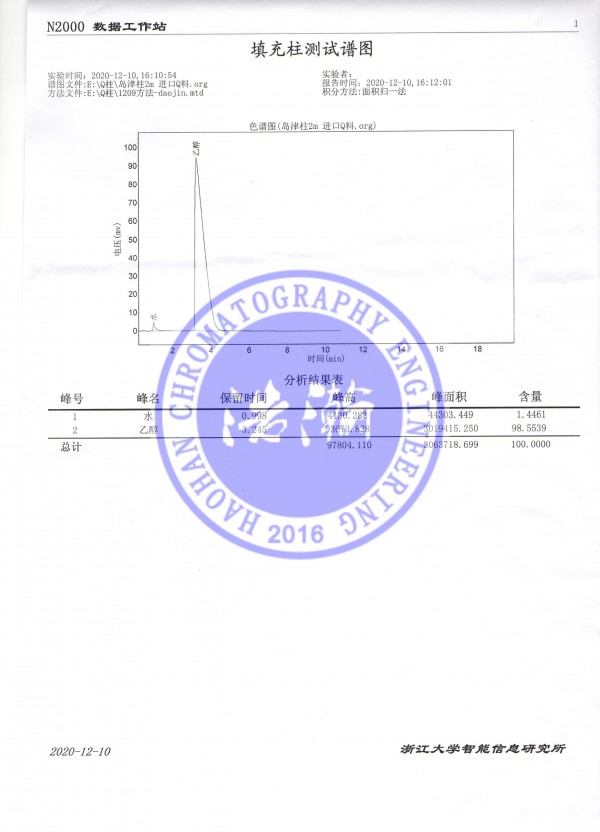



Gas Chromatographic Determination of Water Co
Gas Chromatographic Determination of Water Co
Name: Packed column
Specification: 2m*3mm
Model: Porapak QS
Application: Gas Chromatographic Determination of Water Co
The amount of moisture in the medicine directly affects the quality of the medicine. For this reason, the Pharmacopoeia stipulates water co
Tengzhou Haohan Chromatography Instrument Technology Service Co., Ltd. keeps pace with the times and adopts Porapak QS packed column to determine the water co